7.1-7.6 are likely NOT relevant for passive surveillance components. But for any component design, readers should refer to 7.7 and 7.8.
At this step, a surveillance designer should carefully consider how the study population is going to be selected. Should a census be performed (testing all animals in the target population) or is the population going to be subjected to some type of selective sampling strategy? Even if a census is to be performed, one may still want to consider some information in this section, such as the number of units in the population, as they are important to plan data collection. Moreover, if the target units are clustered (for instance animals clustered in farms), and a census at the top level will be performed (census of herds for instance), sampling within clusters (animals within farms) may still need to be designed.
7.1 Point of sample collection
Addressed in the surveillance components overview. Please see page “2- Overview of surveillance components”.
7.2 Selection of units: census or sampling
Sampling is the focus of the next section. However, the selection of units (whether all the target population defined can be reached - census - or a sampling design will be applied) should consider carefully the availability of a sampling frame. If it is not possible to identify and locate farms/herds/flocks, a surveillance designer should consider where else animals can be located and sampled.
7.3 Target unit level
The target unit is the level of the population for which conclusions will be drawn (for instance animal or herds).
Before sampling a population, a target unit should be define which is the unit of interest when reporting results and drawing conclusions. Target units may be either groups or individuals, and the choice will depend on the aim of the surveillance component. For instance, the target unit will be “herd” if one wants to detect infected herds, estimate the prevalence of infected herds in a region or be able to declare all herds in a given area free from disease. It will be “animals” if one wants for instance to detect cases of disease, estimate the prevalence of a disease in a given animal population or declare a wild population free from disease.
 Framework details: The following options are considered the RISKSUR design framework (drop-down list)
Framework details: The following options are considered the RISKSUR design framework (drop-down list)
- Animal
- Pen
- Group/batch
- Herd/farm
- Hive
- Apiary
- Other
7.4 Sampling unit - individual or group
Sampling units are the units that will actually be sampled.
a) INDIVIDUALS: Individual animal samples
b) MULTIPLE GROUP SAMPLE: Collective/pooled samples which represent multiple animals, but not the entire target unit referred to above (for instance you will take one sample per pen, but target units are expected to be composed of multiple pens; bulk milk samples for more than one group of cows per herd, etc)
c) 1 SAMPLE PER GROUP: Collective/pooled samples which represent the entire group referred to in your target unit (for instance, if your target unit is a pen, and you will take one environmental sample per pen; or one bulk milk sample per herd).
7.5 Sampling design
Based on the decisions outlined above, a surveillance designer should determine whether one or two (or multi-) -stage sampling will be employed:
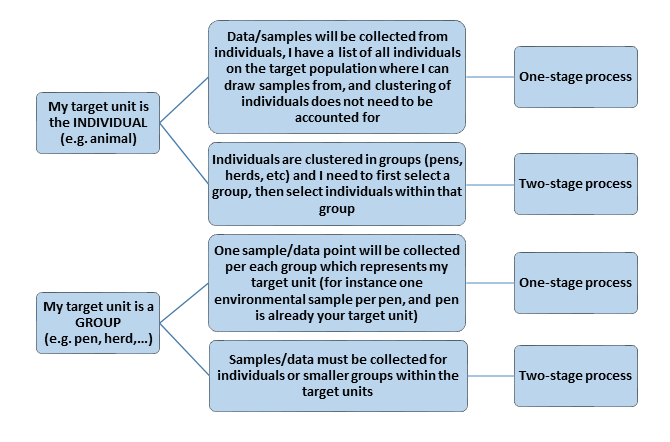
In case of multi-stage sampling (assuming only two stages are needed), the “primary sampling units (PSU)” represent the groups into which the smaller units are organized (such as the herds, slaughter batches, etc.). This will for instance be herds if the goal is to be able to declare all herds in a given area free from disease. The “secondary sampling units” are the individuals within that group, for instance the animals or pens.
7.6 Number of units in the target population
The surveillance designer should document what is know about the number and presence of a sampling frame to the primary sampling units (if using two-stage sampling), or the target units if one-stage sampling is being used. This number may not be defined beforehand, for instance if the sampling population is slaughter batches. In any case as many details as knows should be documented.
The sample should be done for the secondary sampling units. In this case, however, the number of units per primary unit is likely not known or not fixed (for instance number of animals per farm). Again, as many details as possible should be documented, such as for instance how these are defined, if a sampling frame is expected to be available, etc. Normally the number of units per group varies, so a designer shouldn’t be worried about having “a” number of secondary units per primary unit, and the the sample size calculation (later steps) several sizes of secondary sampling units per cluster (PSU) will have to be accounted for.
7.7 Sensitivity of the testing protocol
 __**Useful tools for Sensitivity and Specificity calculation (LINK)**__.
__**Useful tools for Sensitivity and Specificity calculation (LINK)**__.
In order to define the sensitivity, two main factors should be taken into consideration: whether one or multiple tests are used (testing protocol defined in section 6), and whether one- or two-stage sampling was employed (7.5 above).
The sensitivity of the testing protocol will give the sensitivity of detection for the sampled units. In a a study design that involves two stage sampling, the sensitivity at the higher level (primary sampling units, for instance herd) will be given by the sampling strategy, which is defined later in the sample size calculation. For the testing of the units of observation (secondary sampling units), the sensitivity is given by the testing protocol.
Sensitivity for one single test should be given by the laboratory (or literature).
Sensitivity for tests applied in parallel (animal is positive if either test is positive) is calculated as: Sensitivity of test A + Sensitivity of test B – (Sensitivity of test A * Sensitivity of test B).
If tests are applied in series (an animal is only tested with the second test if positive on the first, and only considered positive if positive on both tests) is calculated as: Sensitivity of test A * Sensitivity of test B.
7.8 Specificity of the testing protocol
 __**Useful tools for Sensitivity and Specificity calculation (LINK)**__.
__**Useful tools for Sensitivity and Specificity calculation (LINK)**__.
Specificity for one single test should be given by the laboratory (or literature).
Specificity for tests applied in parallel (animal is positive if either test is positive) is calculated as Specificity of test A * Specificity of test B.
If tests are applied in series (an animal is only tested with the second test if positive on the first, and only considered positive if positive on both tests) is calculated as Specificity of test A + Specificity of test B – (Specificity of test A * Specificity of test B). Alternatively, in surveys to demonstrate disease freedom, where all positives are followed up until proven to be false positives, the assumed specificity is 100%.











